| Medical Device Regulation |
| |
Central Advisory System Sdn Bhd, Malaysia is the pioneer who provides consultancy & training to local and oversea industries on medical devices requirements. |
| |
Background: Cabinet Decisions |
| |
- Cabinet approval on the development of Medical Device Regulatory Program: 16 Feb 2005
- Development of MD Bill & subsidiary legislations
- Establishment of an organization to implement
- Development of MD Registration & Surveillance/Vigilance System
- Establishment of Medical Devices Bureau: August 2005
|
Proposed Medical Device Regulation |
| |
- Aims of regulation
- Scope of regulation
- Elements of regulation
- Definition of medical device
- Classification of medical device
- Safety and performance of medical device
- Good manufacturing principles
|
- Packaging and labeling
- Conformity assessment
- Pre-market requirements
- Requirements for placement on the market
- Register
- Post-market requirements
- Export certificate
- Enforcement and investigation
- Ancillary matters
|
|
Aims of regulation |
| |
- Ensure public health and safety
- Assurance for safety and performance
- Timely access for beneficial medical technologies
- Prevent dumping ground for unsafe and defective medical devices
- Facilitate trade and industry
- Conducive environment for medical devices manufacturing
- Facilitate trade and export
- Promote health tourism
|
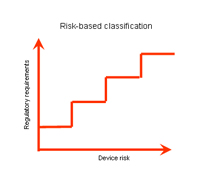
Medical device classification |
| |
Classification of medical devices:
A medical device shall be classified in accordance with the level of risk it poses |
 |
|
| |
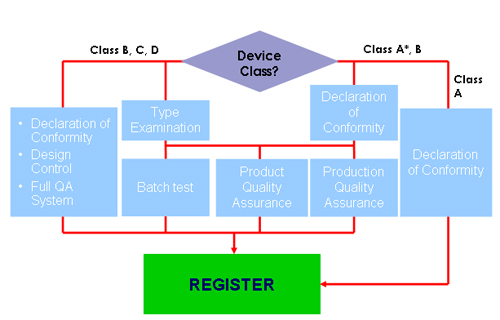
Conformity Assessment Procedure |
| |
 |
| |
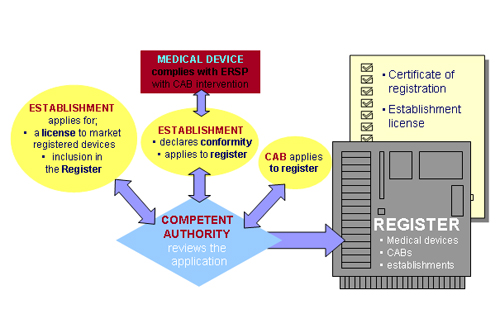
| Register |
| |
 |
| |
| New Requirement |
| - MEDICAL GEAR TO BE OF TOP GRADE |
| |
|